Chemistry of Soaps and Salts – How does soap work?
Those who are reading this topic should have prior knowledge of acids and bases and certain reactions like neutralization reaction.
Salts are compounds that are formed as a product of the neutralization reaction of an acid and a base. There are certain properties of salt, which are –
- Salts have a comparatively higher boiling point and stronger bond.
- They are electrically neutral.
- As they are neutral, they have an equal number of cations and anions present.
- Most Salts are solid at room temperature and easily dissolve in water.
When they are in their solid-state, salts arrange themselves in a rigid structure. That structure is called Lattice.

The most used and known salt is Sodium Chloride, i.e, NaCl. It is also known as Table salt. This is the salt responsible for making ocean water taste salty and also this is the salt we use as our edible salt. Salt is necessary for a living being and also it makes food taste better when salt is added.
Now, as soap is also formed by making an acid react with a base, it is also considered to be a salt.
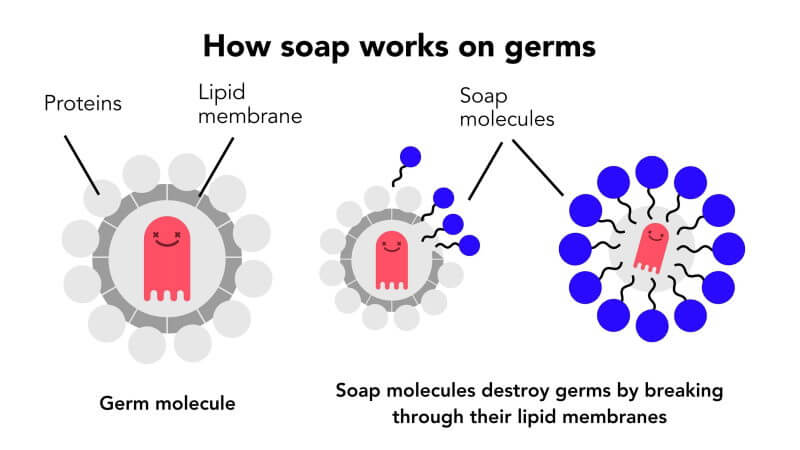
Detergents have special molecules that have two parts, one is called the hydrophilic part and the other is called the hydrophobic part. The Hydrophilic heads are attracted to the water whereas the Hydrophilic Tail attracts grease and dirt. The molecules break the grease or dirt molecules into smaller particles so that they can be cleaned by water.
For many ages, we have been manufacturing soaps using animal fat, water, and ashes, until now. Now with the advancements of technology, we have other ways to form soaps too.
Nowadays, Sodium hydroxide and fats are used to form soaps. Soap forms as the by-product of the reaction along with glycerol. Colors and Fragrances are added afterwards. This process is known as Saponification Reaction.
Some Facts about Salts
- As mentioned before, Glycerol is produced during saponification. This compound is used in the industrial sector to manufacture plastic and explosives.
- There are many salts that are used for the manufacturing of glass.
- The harvest of Natural salt dates back to at least 6000 BC!






Responses