The History of Atom
The Atom is the smallest unit of a substance or a matter. Every solid, liquid, gas is made of atoms. Atoms are extremely tiny. Their size lies around 100 picometer which is equal to 10000000000 meters! So you can imagine how tiny they are!
Now if you ask who discovered Atom, the answer will have names of multiple scientists. There was more than one scientist who was responsible for everything we today know about this tiny particle.
Around 400BC, the Greek philosopher whose name was Democritus was the first person to use the word ‘Atom’. The word ‘Atom’ came from the Greek word ‘atomos’, which means indivisible. He believed that if we keep dividing a piece of matter continuously, a point would come when you will not be able to divide it anymore. This basic unit was what Democritus called Atom.
He called it Theory of the universe. This theory stated the following five points:
- All matter consists of atoms, which is the smallest part of matter too small to be seen.
- There is a space between atoms.
- Atoms are completely solid.
- Atoms have no internal structure.
- Each atom (of a different substance) is different in size, weight, and shape.
FUN FACT: Every person has around 7 billion billion billion atoms in the body, yet you replace about 98% of them every year!
Now let’s come to the 1800s. John Dalton was the first scientist who adapted Democritus’ Theory into the first modern atomic model.
According to John Dalton’s Atomic Model –
- All matter consists of tiny particles called Atom.
- Atoms are indestructible and unchangeable
- Elements are characterized by the weight of their atoms
- When elements react, it is the atoms of the element that have combined to form new compounds.

Now coming to the 1890s. JJ Thompson is the physicist who discovered what we know today as ‘electron‘. To discover it, he used the cathode ray tube technology.
- He took a tube which he nearly emptied, that is the air was sucked out as much as possible.
- An electric charge is passed through the tube. Travels from cathode to anode. (Anode is a conductor that attracts all the negative charges and Cathode is a conductor that attracts all the positive charges.)
- Now, these charges are invisible but Thompson wanted to study and see the charges. So he set up a fluorescent screen is placed at the back of the tube. A dot appears on the screen whenever the beam hits.
- He set the beams in a way that beam will always travel straight if not interfered with.
- The deflection coils each have a specific charge. One is positive and the other is negative.
Through this experiment, Thomson showed (as in the diagram above) that the charge would deflect away from the negative coil. He then said that the charge was therefore negative.

He stated that his charge was 1000 times lighter than a hydrogen atom. He made a statement stating that the negative charge must be inside an atom. This negative charge which he called ‘corpuscles’, later became known as the electron.
FUN FACT: Even after the atoms being the smallest unit of an element, they consist of even tinier particles called quarks and leptons. A lepton is an electron. Protons and neutrons consist of three quarks each!
Now, coming to the years around 1910. Earnest Rutherford was not convinced by Thompson’s model. So he set up an experiment of his own called ‘Gold Foil Experiment’.
In this experiment,
- He fired alpha particles (positively charged) at a gold foil.
- He measured the deflection as the particles came out the other side.
- Most of the particles did not deflect at all.
- The particle deflected back now and then.
- He said that there must be a positive center of the foil. He called this center the nucleus.
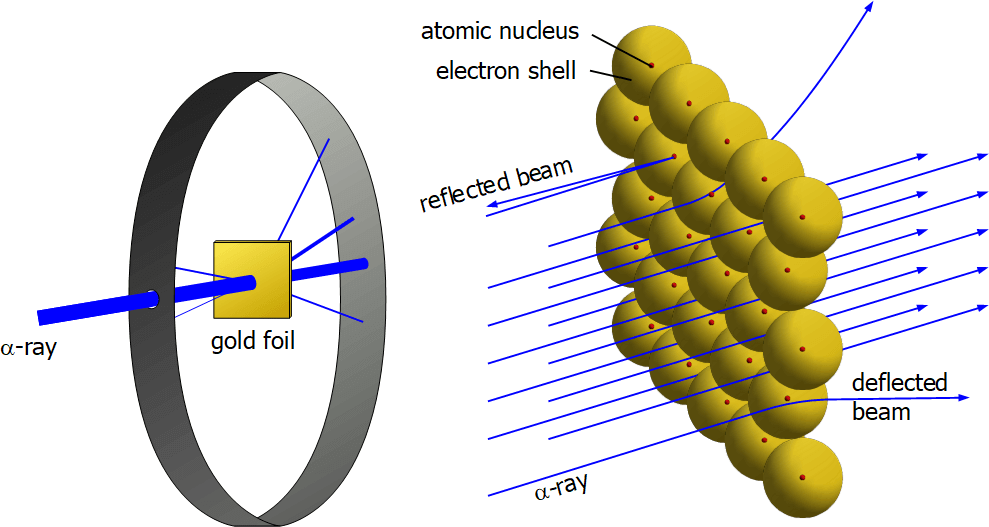
So, Rutherford’s Atomic model stated the following:
- The nucleus of the atom is a dense mass of positively charged particles.
- The electrons orbit the nucleus
- A problem raised was: Why are the negatively charged particles not attracted by the positively charged nucleus
- Rutherford stated that the atom was like a mini solar system and that the electrons orbited the nucleus in a wide orbit. That is why it is known as the planetary model.
FUN FACT: The largest atom (cesium) is approximately nine times bigger than the smallest atom (helium)!
Now around the same time, there was another Scientist called Neil Bohr. He admitted that the Planetary model made by Rutherford was right but it also had its flaws. Using his extensive knowledge in the fields like Quantum Physics and energy, he was able to make Rutherford’s model flawless. One of the major questions Rutherford’s model had was why the electrons did not collapse into the nucleus. Bohr was able to answer that.
So, According to The Rutherford-Bohr Model –
- Electrons orbit around the nucleus in orbits that have a specific size and energy.
- The lower the energy of the electron, the lower is the orbit.
- This means that as electrons fill up the orbitals, they will fill the lower energy level first.
- If that energy level is at maximum capacity, a new energy level will begin.
- Radiation is when an electron moves from one level to another.
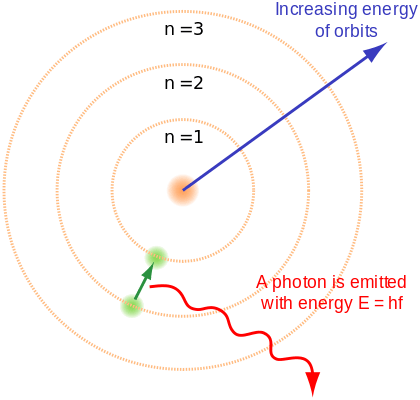
But still, his theory had one last problem which was that according to Bohr’s theory, Electrons do not travel on a specific orbit or path.
FUN FACT: A human hair is nearly 100 nanometers across and that is about 1,000,000 carbon of atoms.
Finally, Around the years of 1920s, Erwin Schrödinger, a revolutionary physicist came up with the atomic model which we still use today.
According to Schrödinger’s model which is also known as the cloud model,
- An Electrol does not travel in an exact orbit.
- We can only possibly predict where it can be.
- We cannot for sure say where it is, we can surely say where it would be.
- The probability depends on the energy level described by Bohr.
So, finally, we can conclude that,
- The smallest unit of a substance or a matter is called an atom.
- Based on structure, each atom is different from other atoms.
- An atom can be divided into subatomic smaller particles called Protons, Electrons, and Neutrons.
- The nucleus is the center of an atom.
- Electrons occupy a certain energy level of a particular.
- A new level begins once the energy level is full.
FUN FACT: The mass of a proton is essentially 1,840 times greater than the mass of an electron but is same as that of a neutron!





informative!