What is the difference between a solid, liquid, and gas?
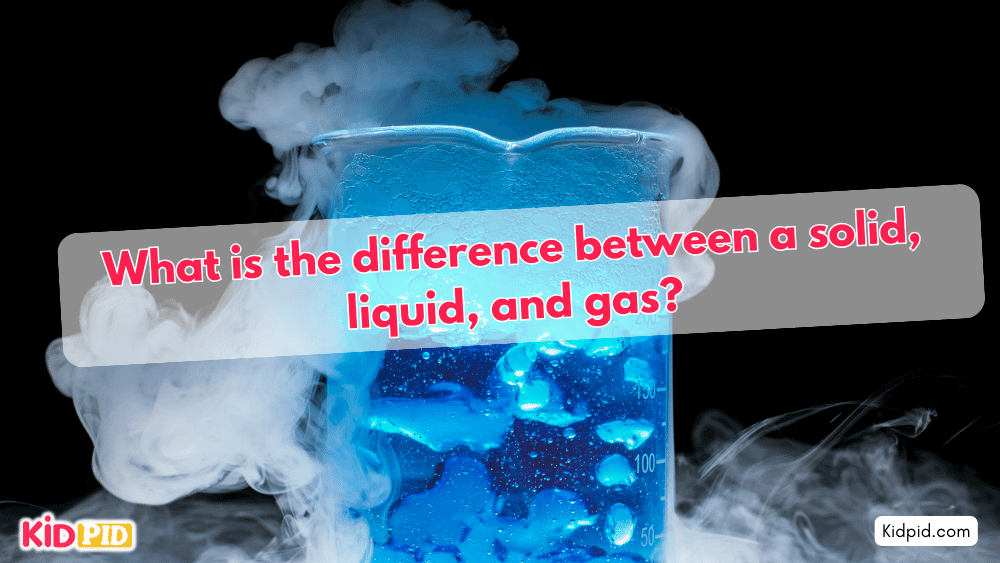
A solid has a definite shape and volume due to closely packed particles that vibrate in place. A liquid has a definite volume but takes the shape of its container because its particles are close but can move past each other. A gas has neither a definite shape nor volume, as its particles are far apart and move freely in all directions.
Contents
MCQs
1. In which state of matter do particles flow and take the shape of their container?
A) Solid
B) Gas
C) Liquid
2. Which state of matter has neither a definite shape nor a definite volume?
A) Solid
B) Liquid
C) Gas
D) Plasma
3. How do particles in a solid move?
A) They do not move at all.
B) They vibrate in fixed positions.
C) They move freely.
D) They flow past each other.
4. What causes a liquid to take the shape of its container?
A) The high energy of the particles
B) The fixed positions of the particles
C) The ability of the particles to move past each other
D) The absence of any attractive forces between particles
5. What characteristic is unique to gases compared to solids and liquids?
A) They have a definite volume
B) They have a definite shape
C) Their particles are closely packed
D) Their particles are far apart and move freely
Read More



Responses