Types of Metals & Alloys
Certain chemical compounds have some distinctive properties like –
- These compounds are conductors of electricity
- They have a lustrous body.
- They have a high melting point
- They are sonorous
These compounds are known as metals. When one or more metallic compound/s are mixed, it’s called an Alloy. The most common example of an alloy is Steel.
Most metals can be found in their solid-state when they are at room temperature, but just like everything, an exception exists here too. Mercury, also a metal, is found to be in its liquid phase at room temperature. Examples of metals are tin, gold, lead, silver, zinc, and so on.
Contents
What are common in these metallic compounds?
- These compounds are heavy and they only melt when they are heated at a very high temperature.
- These compounds are sonorous, that is, they make a bell-like sound when someone hits them.
- These compounds are conductors of heat and electricity. That is, heat and electricity can pass through them easily without obstruction.
- They are malleable, that is a chunk of metal can be beaten into a thin sheet.
- They are ductile, that is, they can be formed into a thin wire.
- These metallic compunds have high tensile strength, i.e., they have a high elasticity which means even if we push or pull them with all our strength, its shape will not change.
Applications
- Metals are easy to shape and also as they are strong, they are used to build different kinds of tools.
- Ships, buildings, and bridges are built using metals and metal alloys like iron and steel.
- Metals like copper, aluminium, gold, silver, and nickel are used to manufacture items that people use for a long time, like coins and electrical wires.
- Sharp objects like Blades, knives, and scissors are made using steel alloy as they remain sharp for a long time.
- Gold, silver, and platinum are metals that have a very high value, for which, they are used to make jewellery.
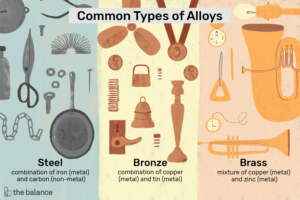
Alloys
Alloys are a mixture of one or more metallic compounds. Some of the most common alloys and the metals whose mixture it is are –
- Steel – A mixture of Iron(Metal) and Carbon(Non-Metal)
- Brass – A mixture of copper and zinc
- Bronze – A mixture of copper and tin
- Gunmetal – A mixture of Copper, Tin, and Zinc
Alloys are made by combining a pure metallic compound with one or more non-metallic or metallic compounds. Except for Strength, the other physical characteristics might not differ much.
Fact: The first-ever discovered alloy was bronze. It was discovered during the prehistoric period. That is why the period is still known to us as the Bronze Age.
Alloys don’t have a single melting point. They have a range for their melting points. There are two terms associated with the melting point range which determines the start and end of the range. Solidus means the temperature at which the melting starts and Liquidus is the temperature at which the melting finishes.






Responses